A clinical trial offers patients the opportunity to receive an investigational treatment, while also supporting progress in scientific research that may eventually help others. Understanding the clinical trial journey is an important first step in considering whether participation in a trial may be right for you. This three-part series will help you:
What gene therapy is designed to do
Investigational gene therapy, specifically adeno-associated virus (AAV) gene therapy for hemophilia, is designed to deliver a functional copy of the F8 or F9 gene into the target cell.
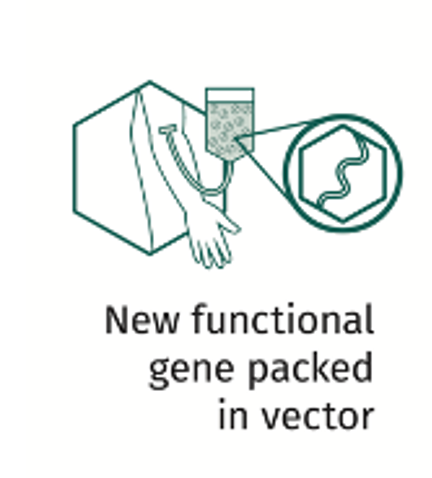
An AAV is used as a delivery vehicle. The viral genetic information is removed, leaving the outside shell (capsid), and a new functional gene is placed inside the shell. This is now a vector.
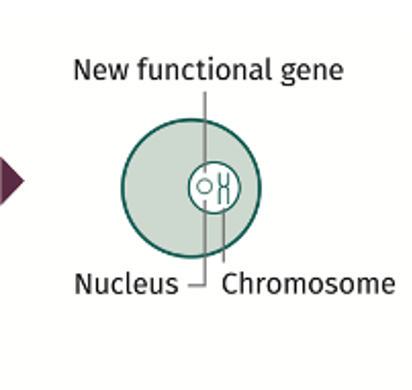
The vector with the new functional gene has an affinity (tropism) for the liver cell (hepatocyte). The vector is intended to travel to the nucleus of the target cell.
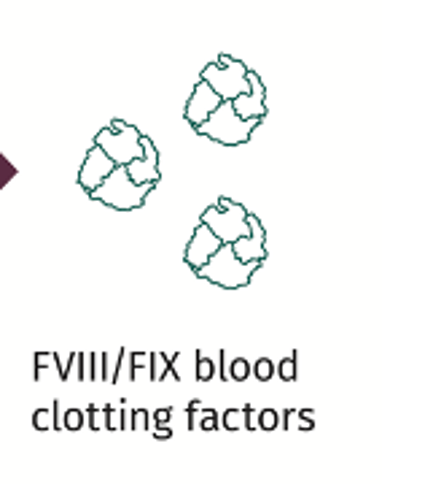
If transferred successfully, the functional gene is intended to provide the correct instructions for the cell to make factor VIII or factor IX clotting-factor proteins. Let’s look at why this is important for people with hemophilia.
The aim of investigational gene therapy for hemophilia
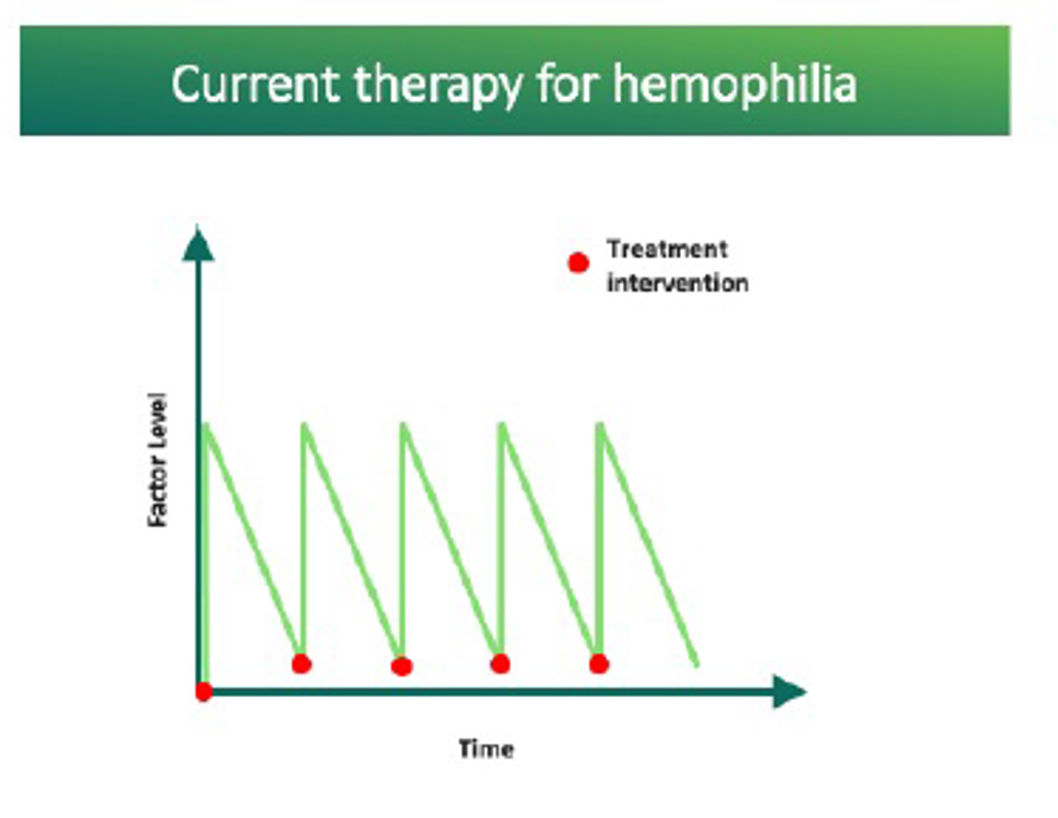
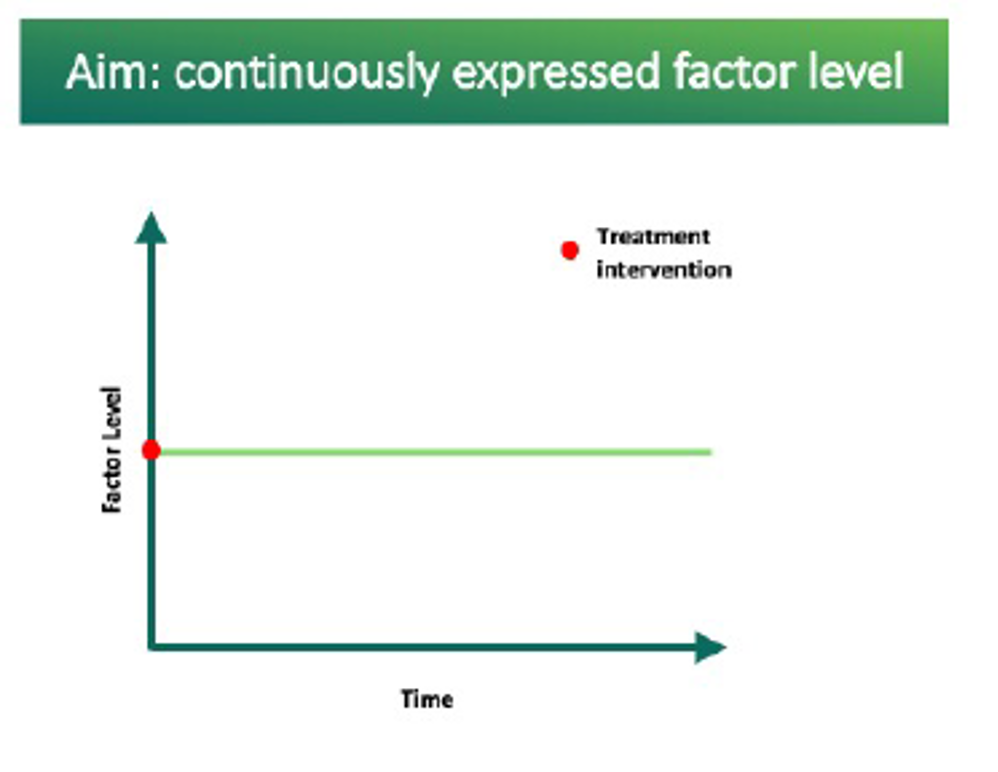
Current treatments require frequent interventions resulting in highs and lows in clotting factor levels (often called “peaks and troughs”). The aim of investigational AAV gene therapy is to move towards the potential of a one-time intervention. This would provide continuously expressed clotting-factor levels.
Talking to your doctor
Investigational gene therapies are being researched for hemophilia as a potential one-time-only dose. That's why you should have informed discussions with your doctor, as it
may be a one-time-only decision. To help you get started, this guide from the National Hemophilia Foundation has additional questions to ask your doctor.
"Investigational" is a term that refers to a potential treatment being studied in clinical trials but isn’t approved by regulatory agencies, such as the U.S. Food and Drug Administration (FDA), as safe and effective for use. In clinical trials, scientists are studying whether the investigational therapy is safe and effective, how much of the medicine may be needed, and how it may be used for that disease. Regulatory agencies then evaluate the information provided by investigators and make a determination on the safety and effectiveness of the treatment. Please consult with your doctor if you want to know more about participating in a clinical trial.
Come back next month to learn what to consider before deciding to participate in a gene therapy clinical trial. Until then, visit Hemophilia Forward®, and check out frequently asked questions about gene therapy clinical trials that people in the hemophilia community are asking.