At uniQure, we know that people with genetic diseases like hemophilia B may have questions about immunity, antibodies, T cells, and treatment options; current and future.
It is always important to talk to a doctor about any questions.
We recently spoke to Valerie Sier-Ferreira, PhD, a lead scientist in immunology from uniQure’s research group, to address some outstanding questions about the importance of antibodies and T cells to experimental adeno-associated virus (AAV)-based gene therapy.

Antibodies are proteins that circulate in the blood and bind to foreign substances, like viruses. T cells are special types of white blood cells that help the body fight off unwanted foreign substances, like viruses. Antibodies and T cells may prevent experimental AAV-based gene therapy from working.
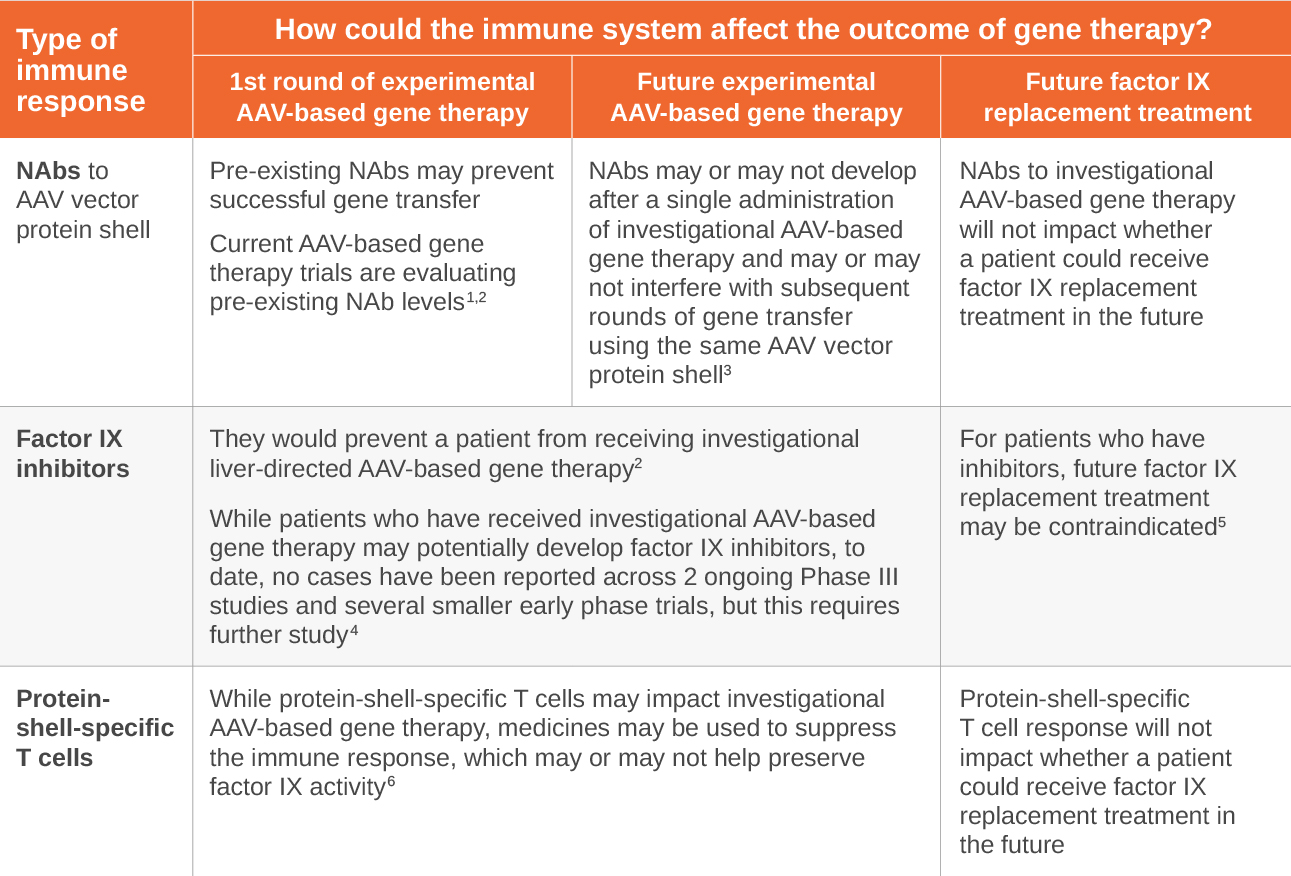
There are different types of AAVs. Most adults have been naturally exposed to AAVs in the environment. The immune system creates antibodies upon exposure that can recognize and neutralize AAVs previously encountered. Experimental AAV-based gene therapy uses protein shells from AAVs to deliver the functional gene to target cells. Some people may have pre-existing neutralizing antibodies (NAbs) that can prevent the AAV vector protein shell from delivering the functional gene.
Factor IX inhibitors are a type of antibody that your body may make that can bind to factor replacement treatment, reducing its ability to help blood clotting and stop bleeding. Only a small percent of people with hemophilia B may develop factor IX inhibitors. If so, this often occurs within the first 50 exposure days to factor IX replacement. While patients who have received investigational AAV-based gene therapy may potentially develop factor IX inhibitors, to date, no cases have been reported across 2 ongoing Phase III studies and several smaller early phase trials, but this requires further study.
In experimental AAV-based gene therapy, the body may have an unwanted reaction to the AAV vector protein shell, which may cause protein-shell-specific T cells to attack target cells. In experimental liver-directed AAV-based gene therapy, a common measurable sign of this response is elevated liver enzymes.
For 13 years, my work at uniQure has been to help develop AAV type 5 (AAV5) as an experimental AAV-based gene therapy. The most rewarding aspect of working with patients with hemophilia is contributing to the development of gene therapies that may potentially improve their future.
Immune responses to AAV-based gene therapy in clinical trials
AAV, adeno-associated virus; NAb, neutralizing antibody
REFERENCES: 1. Gene therapy study in severe hemophilia A patients with antibodies against AAV5 (270-203). ClinicalTrials.gov identifier: NCT03520712. Updated December 23, 2020. Accessed February 3, 2021. https://clinicaltrials.gov/ct2/show/NCT03520712 2. uniQure. Data on file. 2020. 3. Majowicz A et al. Mol Ther. 2017;25(8):1831-1842. doi: 10.1016/j.ymthe.2017.05.003. 4. Batty P, Lillicrap D. Human Molecular Genetics. 2019;28(R1):R95-R101. doi:10.1093/hmg/ddz157. 5. Mingozzi F, High KA. Blood. 2013;122(1):23-26. 6. Carcao M, Goudeman J. Treatment of Hemophilia. 2018;7(5):1-19.
Pioneers in the field of gene therapy
Since uniQure’s start 22 years ago, we have been pioneers, driven to discover gene therapies that have the potential to transform the lives of patients. In the process of obtaining the first regulatory approval for a gene therapy in the European Union, we laid the foundation to become one of today’s industry leaders for researching and developing gene therapies.
Moving gene therapy forward
Understanding the possible challenges that existing and developing therapies face has shaped our current and future research efforts, enabling us to focus on patient needs. uniQure by partnering with patients, is working towards a future where gene therapies may become an important therapy option.
Brought to you by:
©2021 uniQure

