Qfitlia helps your blood's ability to clot by lowering a protein called antithrombin
The U.S. Food and Drug Administration (FDA) recently approved Qfitlia™ (fitusiran) for routine prophylaxis to prevent or reduce the frequency of bleeding episodes in adult and pediatric patients 12 years of age and older with hemophilia A or hemophilia B, with or without factor VIII or IX inhibitors (neutralizing antibodies). The new therapy is manufactured by Sanofi.
Qfitlia is a subcutaneous therapy that uses small interfering RNA (siRNA) technology to target antithrombin (AT), a liver-generated clotting protein that plays a key role in the regulation of blood clots. siRNA molecules are segments of RNA (ribonucleic acid) that block or “silence” the activity of certain genes through RNA interference, a natural biological process common in plants and mammals. It works by silencing the gene responsible for AT, which inhibits the protein’s anticoagulant function. This then compensates for the imbalance caused by deficiencies in other clotting proteins, such as factor VIII (hemophilia A) or factor IX (hemophilia B).
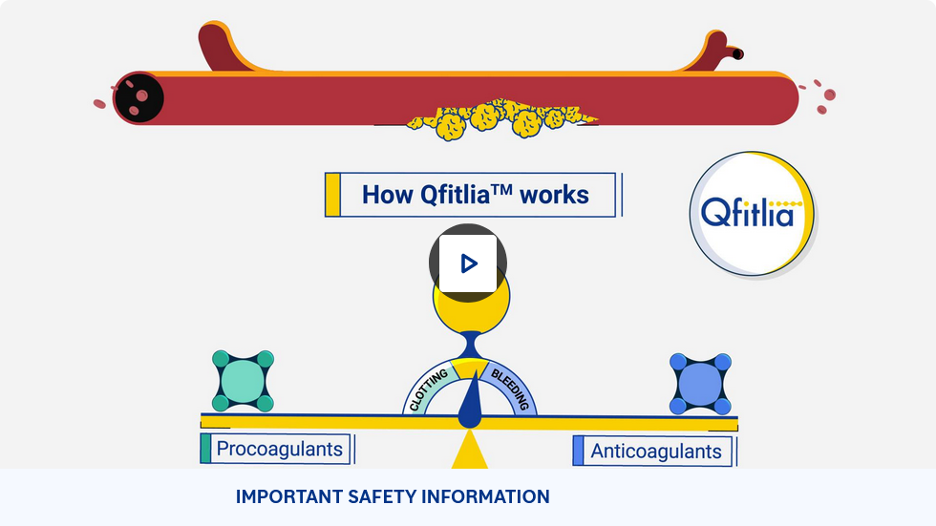
The natural balance between bleeding and clotting is disrupted by hemophilia
“Today’s approval of Qfitlia is significant for patients with hemophilia because it can be administered less frequently than other existing options,” said Tanya Wroblewski, MD, deputy director of the Division of Non-Malignant Hematology in the FDA’s Center for Drug Evaluation and Research. “This new treatment option highlights our continued efforts to improve the lives of patients with hemophilia.”
Our bodies maintain a balance between:
- Procoagulants (like Factor VIII and Factor IX) which help blood to clot
- Anticoagulants (like antithrombin) which prevent blood from clotting
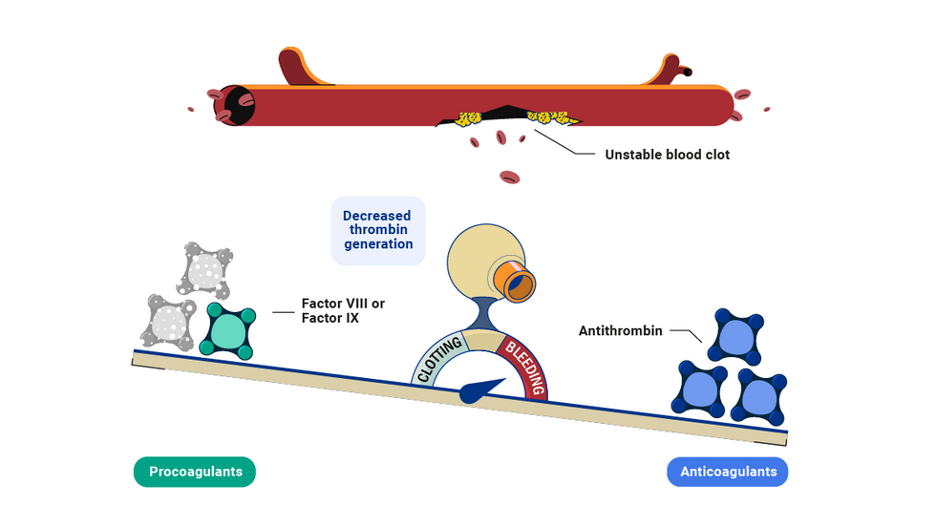
When Factor VIII or Factor IX are missing in hemophilia, this balance is disrupted and thrombin generation decreases.
As a result, an unstable clot forms and bleeding does not stop.
Only Qfitlia helps restore this natural balance by lowering antithrombin
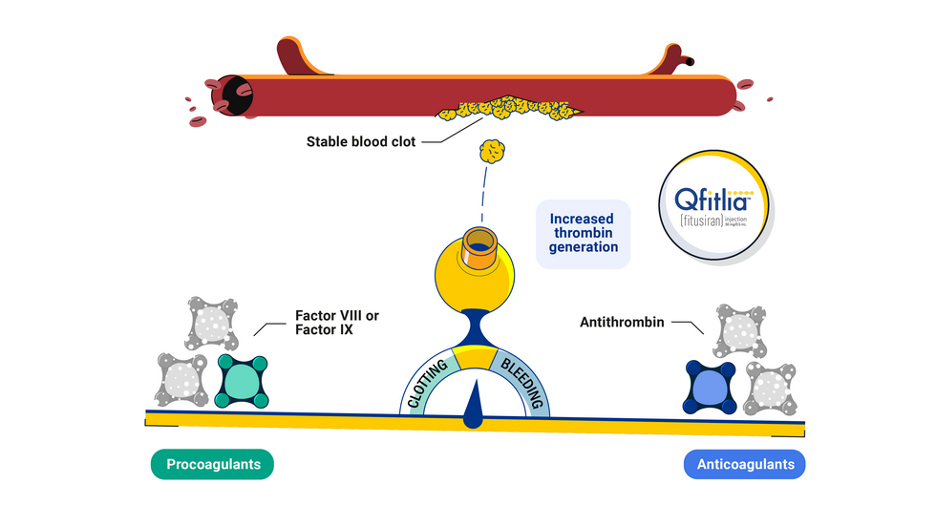
The goal of all hemophilia treatments is to generate more thrombin.
Qfitlia lowers antithrombin to restore balance and increase thrombin generation.
As a result, a stable clot forms.
Procoagulants: Proteins that encourage blood clotting, including Factor VIII or Factor IX.
Anticoagulants: Proteins that prevent blood clotting, including antithrombin.
Thrombin: A protein that is critical to form clots and stop bleeds.
Antithrombin: An anticoagulant that prevents thrombin generation.
Find a Sanofi Educator and connect today
Sanofi Hemophilia Community Relations and Education (CoRe) Managers offer education to people living with hemophilia and their families. CoRe Managers provide information about living with hemophilia and treatment options.
INDICATION
Qfitlia™ (fitusiran) is a prescription medicine used for routine prophylaxis to prevent or reduce the frequency of bleeding episodes in adults and children 12 years and older with hemophilia A or B with or without Factor VIII or IX inhibitors.
It is not known if Qfitlia is safe and effective in children younger than 12 years of age.
* In the clinical study, ~67% of people with hemophilia took Qfitlia every other month, and ~19% took it monthly.