Prophylaxis, a treatment regimen of regular factor infusions, ideally prevents bleeding in the average person with a moderate/severe bleeding disorder. But most patients are far from average. Quinn Packard, 7, of Hampden, Maine, started every-other-day prophylaxis, or prophy, at age 3 to treat his severe hemophilia A. “His bleeds were well managed until age 5,” says his mother, Jill Packard. She is a co-founder of The Hemophilia Alliance of Maine, where she serves as public policy and program manager. “Then he started getting weird, spontaneous breakthrough bleeding in general.” Quinn developed bleeds in his shoulder, elbow, knee and ankle.
In March 2013, Jill took Quinn to the hemophilia treatment center (HTC) in Portland, Maine, for his first pharmacokinetic (PK) study. Pharmacokinetics is the study of how a patient’s body metabolizes, or breaks down, factor product over time. It helps providers see how much infused factor remains after given intervals. A factor’s half-life—or how long it takes for the factor level to drop to half its original concentration—is one of the measurements.
“They told me that Quinn’s half-life was an hour,” Jill says. For someone his age, Quinn’s recombinant product was supposed to have a half-life of around 11.2 hours. By 24 hours, Quinn’s factor level was a mere 6%. His factor dosage for prophy was bumped up by about 1/3, and practically doubled for a major bleed.
But some patients may not need a PK study. Instead, their hematologist may recommend adjusting their factor dose or frequency. It’s important to understand when a PK is called for, what it can reveal and how you can be part of the decision-making process.
PK science
Quinn Packard’s first PK study reveals
the need to adjust his prophy dosage.
Pharmacokinetics (pharmacology + kinetics) is the study of drug activity in the body. It involves drawing blood, plasma, urine, saliva, or other easily sampled fluids in order to measure how a drug is absorbed, distributed, metabolized (broken down), and excreted. A PK study also provides information about a drug’s bioavailability—or how much of it is present in the bloodstream at given time points.
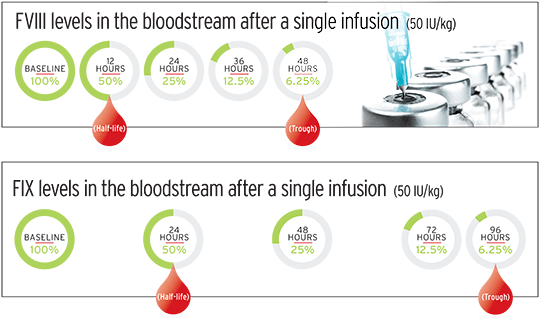
When you infuse a dose of factor, it soon reaches its peak—or maximum concentration—in your blood. That’s when you’re most protected against a bleed. Pharmacokinetics document the half-life of your factor product, typically in 12-hour increments. At about four half-life intervals, or 48 hours after an initial factor VIII (FVIII) infusion, the factor has reached its trough—or minimum concentration. That’s when you’re at your lowest level of protection against a bleed. For factor IX (FIX) infusions, it takes about eight half-lives, or 96 hours, for the factor concentrate to reach its trough level. The next dose of factor product is infused when blood levels have reached zero, or close to it. (See graphic below.)
NikiLitov/Thinkstock
Individual differences
The way your genetic makeup influences how drugs are metabolized in your body is called pharmacogenomics (pharmacology + genetics). For example, two brothers with the same hemophilia gene could have different half-lives based on their pharmacogenomics. “Parents recognize that early on,” says Steven W. Pipe, MD, pediatric medical director of the Hemophilia and Coagulation Disorders Program and director of the Special Coagulation Laboratory at the University of Michigan in Ann Arbor. He is chair of the National Hemophilia Foundation’s (NHF’s) Medical and Scientific Advisory Council (MASAC). “On the same dosing regimen, one boy bleeds more frequently, while the other hardly bleeds.” Your bleeding phenotype, how much you bleed when not on prophy, is also individualized. That explains why someone with moderate hemophilia may bleed more than someone with severe hemophilia.

Age and activity levels affect your bleeding pattern. Children
like Quinn, 7, typically have shorter half-lives than adults.
Your age and activity levels play a role in your bleeding pattern. “Your pharmacokinetics change over time,” says Pipe. “Children under 6 have the shortest half-lives.” They’re generally more physically active and have a higher metabolism, clearing factor from the plasma more rapidly and processing it faster. Quinn’s first PK study at age 5 showed that after one hour, his FVIII level was 59%; at 6.5 hours, it was only 30%. Adults, by contrast, may have sustained factor presence in their blood. “There are some older patients who’ve had half-lives measured pushing 24 hours,” Pipe says.
Having or developing inhibitors—antibodies produced by your immune system that neutralize the effect of your clotting factor concentrate—can also affect half-life. “A really short half-life may be evidence of a clinically undetected inhibitor antibody,” Pipe says. That inhibitor could potentially be missed during a blood test. “My gut feeling is that Quinn had a transient inhibitor back when all this started,” says Jill.
Liver disease, whether a result of chronic hepatitis C virus or another condition, can also affect how your body processes factor concentrate. “FVIII clearance can be significantly delayed if there’s liver disease. That does need to be taken into account,” says Pipe. Liver disease is often associated with elevated factor VIII levels and reduced synthesis of other coagulation factors and proteins.
Even your blood type can influence factor levels. “People with type O blood have lower levels of von Willebrand factor (VWF), the carrier molecule for FVIII,” says Ellis Neufeld, MD, PhD, pediatric hematologist and medical director of the Boston Hemophilia Center. This can result in lower levels of FVIII in the blood. In contrast, having factor V Leiden, a clotting disorder that occurs in 5% of Caucasian Americans, could have the opposite effect. “If you have severe hemophilia and factor V Leiden, you’re likely to have a lower risk of bleeding,” Neufeld says.
Who’s in?
Who qualifies for PK studies is changing. The Boston HTC used to reserve PKs for patients with breakthrough bleeds. “Then it dawned on us that those aren’t the only patients that PK studies would be valuable for,” Neufeld says. To achieve the best treatment schedule, the center now takes PKs on all prophy patients. “ ‘Best’ isn’t the same for every patient, but ‘best’ is what we’re aiming to do,” says Neufeld.
Pipe’s HTC in Michigan takes a different approach. “We tend to tease out the inherent pharmacokinetics in a patient because we do an escalating initiation of prophy,” he says. By starting infants on weekly prophylaxis, then building up to twice weekly and then three times per week, the center can evaluate the impact of infusion frequency and dosing.
That said, there is flexibility in determining who would benefit from a PK study. Breakthrough bleeds in a patient who is receiving optimal dosing would warrant further evaluation. “That’s where a PK study evaluation can be helpful,” says Pipe.
Testing
PKs can be measured at many time points. For FVIII deficiency, typical intervals would be at infusion; then at 12, 36 and 48 hours afterward; and sometimes up to 72 hours later. Quinn’s second PK study in 2015 involved six readings over a three-day period.
But clinicians are questioning the validity of all those measurements. “What we really want to identify is: Does this child have an unusually short or unusually long half-life?” says Pipe. This information can be ascertained using software based on clinical trials. “You can plug that data into these population PK formulas and you can get a pretty accurate assessment of the patient’s pharmacokinetics,” he says. Plus, fewer samples are required. However, the programs are not yet commercially available and the data are often product-specific, says Pipe.
Neufeld is a proponent of “less is more” as well. In some cases, he’ll have the patient infuse at home on a Sunday night, then measure the trough level—the lowest level at which a medicine is present in the body—Tuesday morning, 36 hours later. Two more measurements round out the PK—at next infusion, when factor peaks, and later that week. “We can really learn a lot,” say Neufeld. “Our goal is to get the PK without massively inconveniencing the patient.”
Adjusting doses and intervals

A PK study on JJ Cheeseman provides
his mother with valuable insight.
The goal of prophy is to keep patients’ blood levels of factor protein above 1%, essentially converting people with severe hemophilia to moderate. A 2009 study by lead author Peter Collins and colleagues in the Journal of Thrombosis and Haemostasis confirmed that strategy. Results from 143 kids and adults with severe hemophilia A showed that the longer factor levels dipped below 1%, the higher the total number of bleeds and of joint bleeds.
Still, trough level is a fluid measurement. “The 1% is not a threshold cutoff,” says Pipe. Patients recovering from injury or surgery need higher troughs, he says. So do teens who play team soccer. “Those patients are probably going to be pushed to a more aggressive schedule,” he says. But a 55-year-old sedentary office worker may be fine with a 1% trough. “We adjust the dose and the interval to achieve what’s optimal for that individual patient,” says Pipe.
What’s optimal, though, can change. Quinn’s parents initiated the second PK test in March 2015 because of spontaneous elbow and knee bleeds the night before his next prophy dose. Despite a longer half-life (it had reached six hours), by 24 hours, he only had 6.8% left. So Quinn’s factor dosage was increased both for prophy and major bleed. Six months later, the dosage was upped again to prevent breakthrough bleeds at night.
Extended half-life products
FIX Products
If you are considering the new extended half-life products, Neufeld offers a hot tip from a hematologist: Get a PK assessment done on your tried-and-true product before starting the new extended half-life concentrate. “Some people have a longer half-life on the current FIX product than they might guess,” he says. A 20-hour half-life means you have a decent factor level for up to several days, says Neufeld. On the other hand, you might have a much shorter half-life. If you’re on twice-weekly FIX infusions, your trough level on days three and seven are key. “I’d like to know if the trough is so low that I’m not even protecting them,” he says.
Case in point: 2-year-old JJ Cheeseman, of Buffalo, New York. After NHF’s 67th Annual Meeting in Dallas in August 2015, his mother, Erica, was a mom on a mission. She had attended the educational session, “What is half-life and why do I need to understand it?” and was enlightened. She requested that JJ’s hematologist perform a PK study on the FIX product infused twice weekly for his severe hemophilia B. The family was planning to switch JJ to an extended half-life product. “I wanted to be able to compare his half-life on the new product to what he’s on now,” says Erica.
The PK results revealed that JJ’s FIX level after 24 hours was only 14%, when it should have been 50%. By 48 hours it was zero. This confirmed Erica’s suspicions. “I already know because of the way he’s bruising that he doesn’t have a 50% half-life,” she says. From the PK studies, Erica has gained education and expertise about JJ’s hemophilia. “I’ve learned to understand him—how his bleeds work, how his body works and how much factor he’s OK to have if he hurts himself,” she says.
Now as JJ toddles through the house, Erica more calmly assesses his ups and downs. If he takes a tumble within 24 hours of an infusion, she knows he’s covered. “It takes the worry out when you know how much factor he has in his blood,” she says. Before, on Day 3, she’d assume he had 25% left, but now she knows he’s below his trough. “I know if he needs to get infused,” adds Erica.
FVIII Products
Extended half-life FVIII products typically last 1.5 times as long as prior products, says Neufeld. He anticipates they’ll be most beneficial for two subsets of patients: those with very long current FVIII half-lives, and those on every-other-day prophy who have short half-lives and experience breakthrough bleeding despite frequent infusions. For example, patients with 14-hour half-lives could be looking at 20-hour half-lives. “They’d probably be able to do twice-a-week dosing or every fourth day,” says Neufeld.
PKs and personalized prophy
As a chapter leader, Jill has heard parents’ frustrations over their kids’ recurring bleeds. She has advice for them. “You need to speak up,” she says. “Doctors and parents need to make and take the time for PKs.” PK studies are helping pave the way for personalized prophylaxis, which hopefully translates into fewer bruises and bleeds, and better joint health in the long run.