You might have read our last few articles where we ask a lot of important questions about gene therapy, such as,
- What is gene therapy?
- What are the risks and benefits of gene therapy?
- How do therapeutic vectors work?
The answers to these questions can seem complex. That’s why we set out to find the clearest, simplest way of talking about gene therapy research.
HOW DID WE DO THIS? WE STARTED A CONVERSATION.
We spoke to people with hemophilia, caregivers, and hematologists from around the world. We asked what they thought about certain words or phrases. We asked them how these words made them feel and what they liked or didn’t like about them.
WHAT DID WE LEARN?
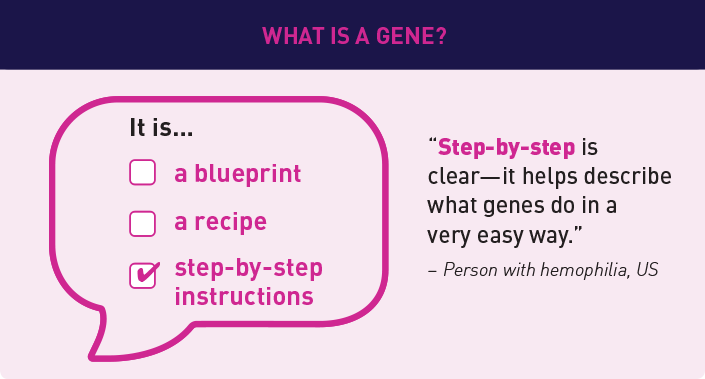
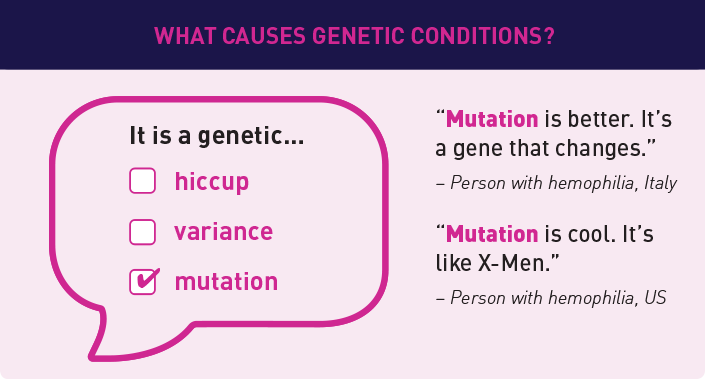
We found that using consistent and common language made understanding gene therapy research a lot easier. Here are a few examples of how we define some of our common terms based on what we heard:
LET'S DIVE A LITTLE DEEPER
During our conversations, we heard that some terms and phrases were less familiar than others. So, let’s talk about them.
What is a therapeutic vector?
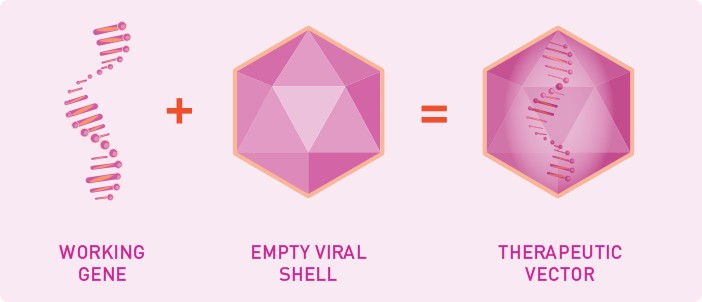
Gene therapy research is investigating whether a therapeutic vector can be used to deliver a working gene into the body. It is designed to protect the working gene DNA and direct it where it needs to go. Therapeutic vectors are made in a lab, where scientists create the outer shell of a virus. The shell contains the working gene DNA inside, instead of the original viral DNA.
- The viral shell used in these vectors is made by modifying a naturally occurring virus. After it’s modified, the viral shell does not contain any viral DNA. So, it doesn’t make people sick
- Scientists make therapeutic vectors from viruses because they are very good at getting to the cells where they’re needed
- Read the full story on how therapeutic vectors work
![]()
What does it mean to be eligible for gene therapy research?
To participate in a research study, candidates must meet certain measures. These are called eligibility criteria. A healthcare provider will determine whether their patient is eligible for gene therapy research by considering factors such as age, sex, and organ health. A blood test may be required to check for antibodies against the virus being used to build the therapeutic vector.
- A type of virus called an adeno-associated virus (AAV) is commonly used in gene transfer. This type of virus occurs naturally and is found around the world. It does not make people sick. Some people who have already been exposed to this virus may have antibodies. This could make certain gene therapies less effective or not effective at all
No gene therapies for hemophilia A or B have been approved for use or determined to be safe or effective.

© 2020 BioMarin Pharmaceutical Inc.
All Rights Reserved. MMRC-GTH-0581 11/20


